
COVID boosters well-tolerated during and after pregnancy
In addition, a practice advisory issued this week says pregnant and lactating individals should get booster jabs.Media Contact: Barbara Clements, 253-740-5043, bac60@uw.edu
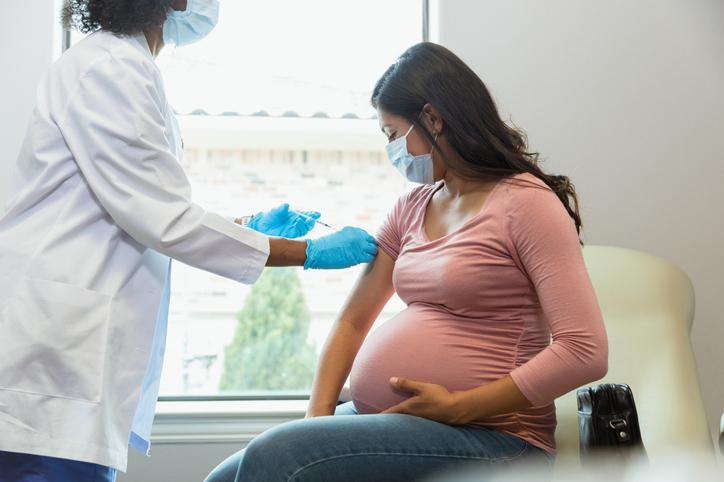
The COVID-19 booster shots are well tolerated by both pregnant and nursing women, a new study published Sept.8 in JAMA Network Open concluded.
The UW Medicine-led study with over 17,000 participants showed that “there were very few obstetric concerns after patients received the boosters,” noted lead author and UW Medicine OB-GYN Dr. Alisa Kachikis.
In addition, the American College of Obstetricians and Gynecologists issued a practice advisory today encouraging pregnant or lactating individuals to receive the latest COVID-19 booster, which has been modified to protect against the BA.4 and BA.5 variants. That booster became available last week.
"This shot is going to be recommended for all individuals who have two months since their last booster," said UW Medicine OB-GYN Dr. Linda Eckert, "And if you're pregnant, that includes you. If you're postpartum, that includes you."
Eckert said that this study backs up the importance of pregnant and lactatimg women getting their boosters. Eckert, who is a professor of obstetrics and gynecology at the University of Washington School of Medicine, was the senior author on the study.
“The majority of pregnant and lactating participants did really well with the COVID-19 booster. In fact, most participants reported that symptoms with the booster or 3rd dose were less severe than symptoms with their initial COVID-19 vaccine series,” Kachikis added.
As such, it’s important that primary-care providers continue to recommend both the initial vaccine series and the boosters to pregnant and lactating women, she said. Findings from this study are especially pertinent now with the new COVID-19 booster becoming more widely available this month, she added.
Kachikis and her team collected data from a follow up survey which was sent out to just over 17,500 participants last October. These participants were part of an ongoing cohort survey to monitor the reaction of pregnant and lactating individuals to both the initial vaccine, and to, in this study, the booster shots.
Just over 97 percent of the cohort completed the follow-up survey on boosters. In this cohort group, 11 percent were pregnant; 60 percent were lactating and 27 percent were neither pregnant or lactating at the time of the survey. Most (82 percent) reported soreness at the injection site; with 68 percent reporting another symptom such as fatigue or fever
Importantly, when it came to a COVID-19 booster or third dose, pregnant participants were significantly more likely to list their health-care professional as an important source for information and to have received a recommendation to receive the booster. This suggests that clinicians may play a significant role in vaccine acceptance and as a source for vaccine information.
The original study, launched in January 2021, was designed to monitor the reaction of pregnant and lactating individuals to the initial COVID-19 vaccinations. The mean age of the group was 33 years, with 92 percent of the group identifying as white and 99 percent identifying as female. When Kachikis designed this online cohort study of women, it included those who were pregnant or lactating and those who were neither pregnant nor lactating.
This follow up study of the online cohort, as they receive booster doses, continues to support the finding that pregnant and lactating individuals tolerate the COVID booster vaccines well, and that they should be included in clinical trials for other relevant vaccines, Eckert said.
Aside from the CDC vsafe registry, this is the largest U.S. study of this issue. Canada has created a registry based on Kachikis' model.
According to the CDC, 71.3 percent of pregnant women in the United States have received at least the primary COVID-19 vaccine series either before or during pregnancy. ACOG estimates 55% have received a booster. This study offers importance reassurance to pregnant and lactating individuals who are due for a booster dose. Kachikis hopes this study, as well as other reassuring studies, will encourage pregnant and lactating women to get boosted, and vaccinated, if they have not done so already.
This research was supported by the National Center For Advancing Translational Sciences (UL1 TR002319) and by the National Institute of Child Health and Human Development Women’s Reproductive Health Research Award (2 K12HD001264-21) for the purposes of design and conduct of the study; collection, management, analysis, and interpretation of the data. The UW Institute of Translational Health Science provided administrative Research Electronic Data Capture support for this project and was supported by grants UL1 TR002319, KL2 TR002317, and TL1 TR002318 from NCATS.
The content of the study report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
For details about UW Medicine, please visit https://uwmedicine.org/about.