
Study: ‘Bionic pancreas’ improves type 1 diabetes control
Users of the investigational device spent 11% more time in the target blood-glucose range than control-group members.Media Contact: Brian Donohue - 206-543-7856, bdonohue@uw.edu
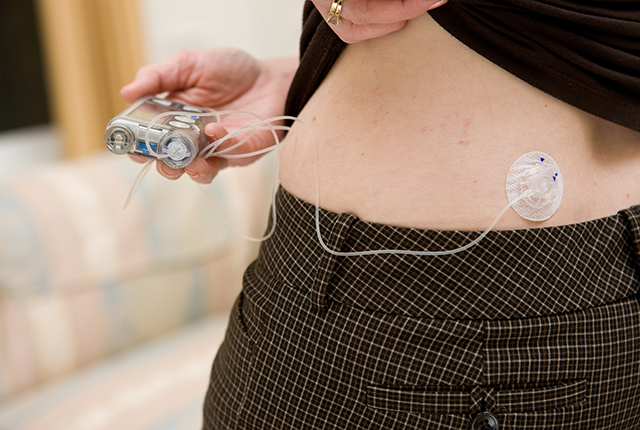
A pocket-size wearable device known as a bionic pancreas, which uses next-generation technology to automatically deliver insulin, was more effective at maintaining blood glucose (sugar) levels within normal range than standard-of-care management among people with type 1 diabetes, a new multicenter clinical trial has found.
Results were published today in the New England Journal of Medicine.
Automated insulin-delivery systems, also called artificial-pancreas or closed-loop control systems, track a person’s blood glucose levels using a continuous glucose monitor; they automatically deliver the hormone insulin via pump as needed. These systems replace reliance on fingerstick tests of glucose levels, glucose monitoring that still requires multiple daily insulin injections, and non-automated pumps.
“This pump uses more artificial intelligence than the other pumps on the market. It identifies trends of the continuous glucose monitor to give insulin that keeps blood glucose in the normal range — without patients having to count carbohydrates,” said Dr. Irl Hirsch. He is a diabetes expert at the University of Washington School of Medicine, one of 16 U.S. trial sites and the only one in the Pacific Northwest.
Compared with other artificial pancreas technologies, the bionic pancreas requires less user input because the device’s algorithms continually adjust insulin doses based on users’ needs. Users initialize the bionic pancreas by entering their body weight into the device’s dosing software at the time of first use.
“Many patients closely manage their type 1 diabetes, and do extremely well,” Hirsch said. “But there’s another, very large group of patients who don’t pay as much attention, for a variety of reasons. Our findings could be informative for those patients, in terms of keeping their blood sugar under better control.”
The 13-week trial enrolled 326 participants ages 6 to 79 years who had type 1 diabetes and had been using insulin for at least one year. Participants were randomly assigned to either a treatment group using the bionic pancreas device or a standard-of-care control group using their personal pre-study insulin-delivery method.
In participants using the bionic pancreas, glycated hemoglobin, a measure of a person’s long-term blood glucose control, improved from 7.9% to 7.3%, yet remained unchanged among the standard-of-care control group. The bionic pancreas group participants spent 11% more time, approximately 2.5 hours per day, within the targeted blood-glucose range compared with the control group. These results were similar in youth and adult participants.
Hyperglycemia, or high blood glucose, caused by problems with insulin pump equipment, was the most frequently reported adverse event in the bionic pancreas group. The number of mild hypoglycemia events, or low blood glucose, was low and was not different between the groups. The frequency of severe hypoglycemia was not statistically different between the standard of care and bionic pancreas groups.
The trial was primarily funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. Funding for the study was provided by NIDDK grant 1UC4DK108612 to Boston University, by an investigator-initiated study award from Novo Nordisk, and by Beta Bionics, which also provided the experimental bionic pancreas devices used in the study. Insulin and some supplies were donated by Novo Nordisk, Eli Lilly, Dexcom and Ascensia Diabetes Care. Partial support for the development of the experimental bionic pancreas device was provided by NIDDK SBIR grant 1R44DK120234 to Beta Bionics.
For details about UW Medicine, please see our About page.