
Stem cell-derived organoids secrete tooth enamel proteins
The advance is seen as a critical first step toward novel treatments to repair and regenerate teeth.Media Contact: Leila Gray, 206.475.9809, leilag@uw.edu
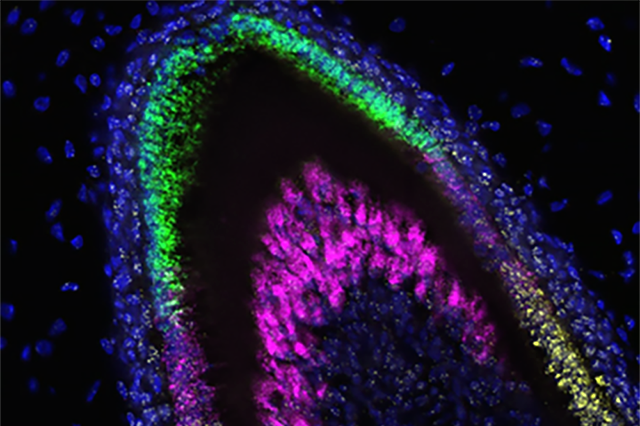
Organoids have now been created from stem cells to secrete the proteins that form dental enamel, the substance that protects teeth from damage and decay. A multi-disciplinary team of scientists from the University of Washington in Seattle led this effort.
“This is a critical first step to our long-term goal to develop stem cell-based treatments to repair damaged teeth and regenerate those that are lost,” said Hai Zhang, professor of restorative dentistry at the UW School of Dentistry and one of the co-authors of the paper describing the research.
The findings are published today in the journal Developmental Cell. Ammar Alghadeer, a graduate student in Hannele Ruohola-Baker's laboratory in the Department of Biochemistry at the UW School of Medicine was the lead author on the paper. The lab is affiliated with the UW Medicine Institute for Stem Cell and Regenerative Medicine.
The researchers explained that tooth enamel protects teeth from the mechanical stresses incurred by chewing and helps them resist decay. It is the hardest tissue in the human body.
Enamel is made during tooth formation by specialized cells called amelobasts. When tooth formation is complete, these cells die off. Consequently, the body has no way to repair or regenerate damaged enamel, and teeth can become prone to fractures or subject to loss.
To create ameloblasts in the laboratory, the researchers first had to understand the genetic program that drives fetal stem cells to develop into these highly specialized enamel-producing cells.
To do this they used a technique called single-cell combinatorial indexing RNA sequencing (sci-RNA-seq), which reveals which genes are active at different stages of a cell’s development.
This is possible because RNA molecules, called messenger RNA (mRNA), carry the instructions for proteins encoded in the DNA of activated genes to the molecular machines that assemble proteins. That is why changes in the levels of mRNA at different stages of a cell’s development reveal which genes are turned on and off at each stage.
By performing sci-RNA-seq on cells at different stages of human tooth development, the researchers were able to obtain a series of snapshots of gene activation at each stage. They then used a sophisticated computer program, called Monocle, to construct the likely trajectory of gene activities that occur as undifferentiated stem cells develop into fully differentiated ameloblast.
“The computer program predicts how you get from here to there, the roadmap, the blueprint needed to build ameloblasts,” said Ruohola-Baker, who headed the project. She is a professor of biochemistry and associate director of the UW Medicine Institute for Stem Cell and Regenerative Medicine.
With this trajectory mapped out, the researchers, after much trial and error, were able to coax undifferentiated human stem cells into becoming ameloblasts. They did this by exposing the stem cells to chemical signals that were known to activate different genes in a sequence that mimicked the path revealed by the sci-RNA-seq data. In some cases, they used known chemical signals. In other cases, collaborators from the UW Medicine Institute for Protein Design created computer-designed proteins that had enhanced effects.
In the course of conducting this project, the scientists also identified for the first time another cell type, called a subodontoblast, which they believe is a progenitor of odontoblasts, a cell type crucial for tooth formation.
The researchers found that together these cell types could be induced to form small, three-dimensional, multicellular mini-organs, called organoids. These organized themselves into structures similar to those seen in developing human teeth and secreted three essential enamel proteins: ameloblastin, amelogenin and enamelin. These proteins would then form a matrix. A mineralization process that is essential for forming enamel with the requisite hardness would follow.
Zhang said the research team now hopes to refine the process to make an enamel comparable in durability to that found in natural teeth and develop ways to use this enamel to restore damaged teeth. One approach would be to create enamel in the laboratory that could then be used to fill cavities and other defects.
Ruohola-Baker points out that another more ambitious approach would be to create “living fillings” that could grow into and repair cavities and other defects. Ultimately, the goal would be to create stem cell-derived teeth that could replace lost teeth entirely.

Ruohola-Baker said teeth are an ideal model to work on the development of other stem cell therapies.
“Many of the organs we would like to be able to replace, like human pancreas, kidney and brain, are large and complex. Regenerating them safely from stem cells will take time,” she said. “Teeth on the other hand are much smaller and less complex.They’re perhaps the low-hanging fruit. It may take a while before we can regenerate them, but we can now see the steps we need to get there.”
She predicts, “This may finally be the ‘Century of Living Fillings’ and human regenerative dentistry in general.”
In addition to researchers from the Department of Oral Health Sciences in the UW School of Dentistry, other scientists from the UW Brotman Baty Institute, the UW Allen School of Computer Science and Engineering, Seattle Children’s Research Institute, Institute for Stem Cell and Regenerative Medicine, Institute for Protein Design, the Department of Engineering in the UW College of Engineering Bioengineering (a joint department in UW’s College of Engineering and School of Medicine), Biochemistry, Comparative Medicine and Pediatrics, Genome Sciences, all at the UW medical school, and the SRM Institute of Science and Technology, Chennai, India, all contributed to the study.
This work was supported by funding from the U.S. National Institutes of Health (1P01GM081619, R01GM097372, R01GM97372-03S1, R01GM083867, 5R24HD000836, T90DE021984, R01DE033016, U01DK127553, R01DK117914), the National Heart, Lung and Blood Institute Progenitor Cell Biology Consortium (U01HL099997; UO1HL099993), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, UW Medicine Institute of Stem Cell and Regenerative Medicine Fellowships and the Dr. Douglass L. Morell Research Fund. Work conducted in the Institute for Stem Cell and Regenerative Medicine’s Genomics Core was supported by a gift from the John H. Tietze Foundation
Written by Michael McCarthy
For details about UW Medicine, please visit https://uwmedicine.org/about.